Research
Space is a demanding environment, and providing reliable power for spacecraft is an ongoing challenge. To help ensure NASA maintains its leadership in advanced space power systems, our team conducts research on a variety of energy storage technologies. In addition to work in support of space exploration, we conduct a variety of research activities in support of terrestrial applications, funded through sponsors including the Department of Energy, the Department of Defense, and private industry.
Project
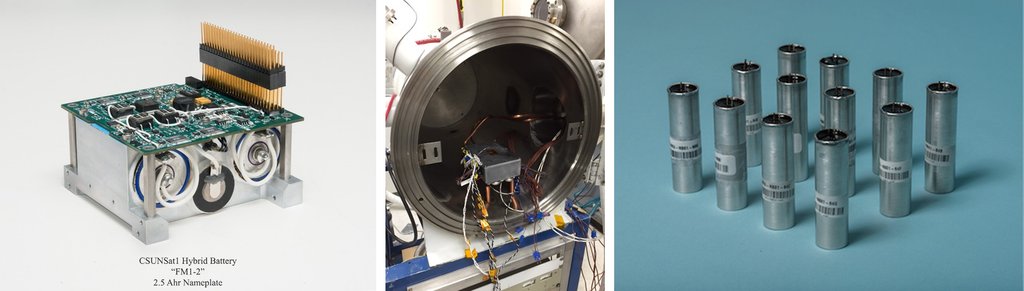
Long life, rechargeable batteries play a critical role in NASA missions. While commercially supplied Li-ion batteries are now widely available, these batteries are often unsuitable for use in the harsh environments of space. Specialized space-rated Li-ion batteries have been developed by JPL and the United States Air Force, in concert with domestic commercial partners, and are now successfully deployed in virtually all NASA missions, including the Mars Exploration Rovers and Juno.
JPL continues to employ a dual strategy of careful selection and extensive qualification of commercially available cells, coupled with the development of custom chemistries for operation in the most demanding environments. Research in this area covers the full gamut of activities, from basic materials research to testing in lab scale cells. Extensive partnering with commercial vendors provides a clear path to scale-up and mission infusion. JPL also partners with external agencies, such as the Department of Energy, to develop electrolytes to support wide temperature operation in electric vehicles.
NASA continues to press the frontiers of exploration, and envisions sending humans to Mars, spacecraft into the atmosphere of Venus, onto the surface of outer planetary icy moons, and even boring through the ice covering liquid oceans on these moons. For such challenging missions, current space-rated Li-ion batteries will not suffice. To that end, advanced Li-ion batteries are being developed at JPL to, for example, extend the operational temperature range, increase the specific energy of the electrodes, and improve the radiation tolerance of space-rated Li-ion batteries.

In the 1970s and 1980s, JPL developed and evaluated fully electric vehicles powered by the most advanced batteries of the day — decades before the current revolution in electric cars. Today, work at JPL focuses on the development of advanced electrolytes that are optimized for the harsh environment "under-the-hood." All of the programs have been supported by the Department of Energy.
The deposition of materials using electrochemical means (electroplating, or electrodeposition) plays an increasingly important role in numerous technology areas. Various JPL technology development efforts require electroplating, due to the need for the deposition of novel, thick film materials, or due to the use of a complex, underlying shape that does not lend itself to traditional physical deposition techniques.
The JPL Electrochemical Research, Technology, & Engineering Team is often called upon to utilize its expertise in electrodeposition, to support these development efforts. Previous efforts include the development of advanced micro-thrusters, as well as miniaturized power magnetic devices such as inductors and transformers, for highly integrated power electronics. Finally, advanced sputtering techniques are used to deposit solid state electrolytes, for the fabrication of highly miniaturized, planar battery cells.
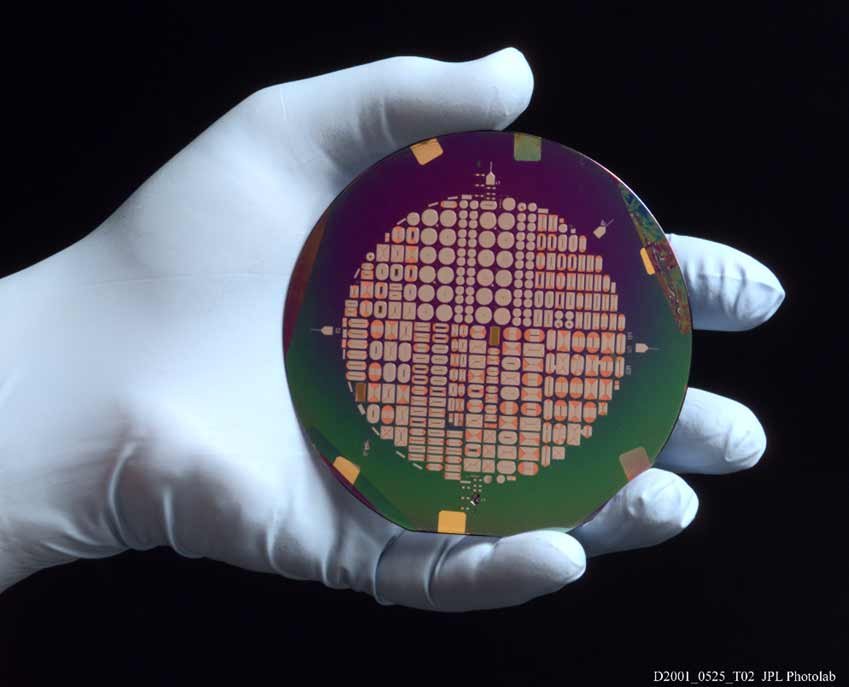
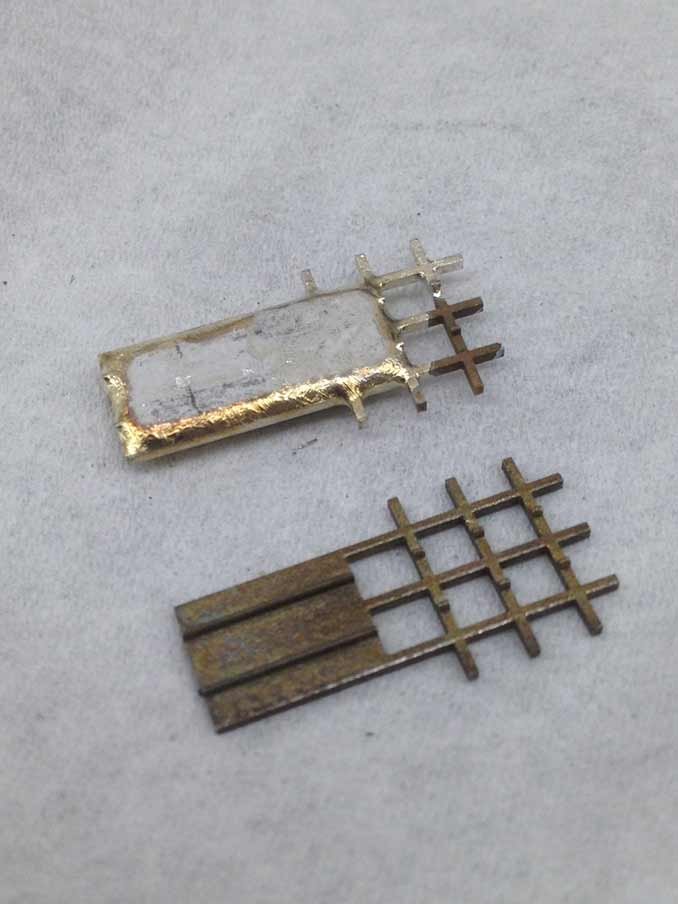
Planar micro-inductors fabricated on a silicon wafer using electrodeposition. These parts feature electroplated Permalloy magnetic cores and copper windings, patterned using standard photolithography techniques. They were developed for application in miniaturized dc-dc converters for use in small spacecraft.
JPL's Electrochemical Research, Technology, & Engineering Team research and technology development efforts include the development of fuel cell and electrolyzer technologies for space, military and commercial applications. Fuel cell technologies under development have included direct methanol fuel cells (DMFC), proton exchange membrane (PEM) and alkaline exchange membrane (AEM) fuel cells. These development efforts span a wide range of activities from high throughput materials screening for catalyst discovery, system-level modelling and design, membrane-electrode assembly fabrication and testing, and integration of fuel cells with rechargeable batteries. Current effort focus on a next generation of primary fuel cell systems, capable of supporting outer planets exploration and long duration surface operations.
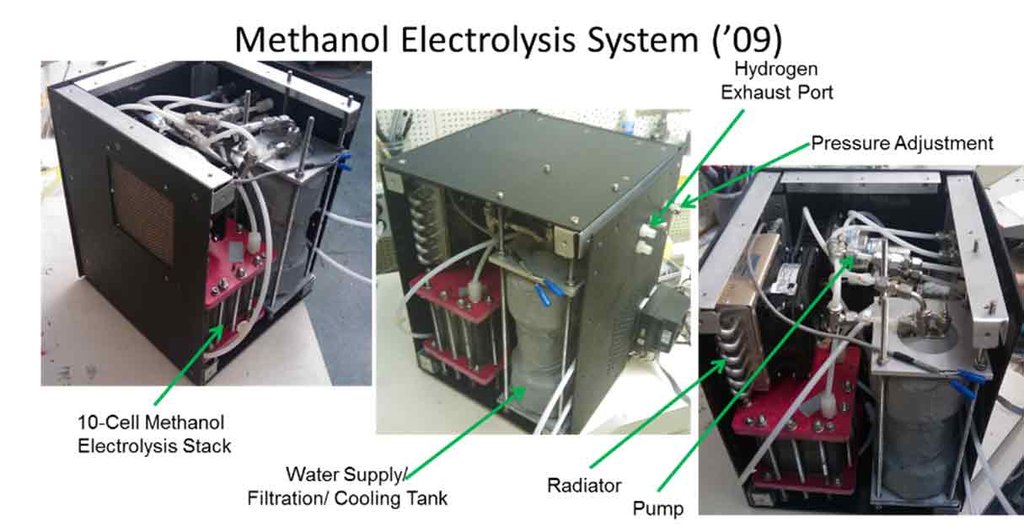
Much of the focus is on portable power systems, capable of operating in harsh environments. Recent efforts have focused on the design of compact, portable regenerative fuel cell systems, to support large rovers and even human operations on Mars. The team is developing portable regenerative fuel cell systems (RFCs) that have targeted NASA applications, but can also be deployed terrestrially. These RFC systems are modular and can accommodate a range of energy storage configurations. Hydrogen generation via water and methanol electrolysis is also a key development effort, particularly in the area of high pressure electrolysis.
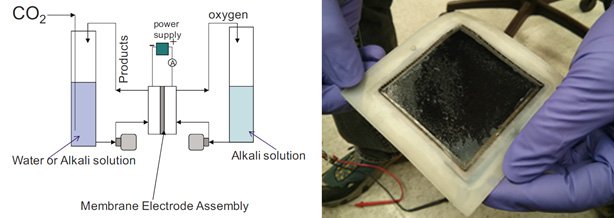
The human and robotic exploration of the solar system presently is limited by the amount of resources that accompanies the spacecraft. Long-term exploration will require humans and robotic spacecraft to "live off the land", generating resources such as fuel, water, and food from in-situ sources. JPL's Electrochemical Research, Technology, & Engineering Team is engaged in ISRU research, such as developing electrochemical methods for converting carbon dioxide to fuels. Efforts in this area have focused on the development and demonstration of prototype test cells, as well as the development of cell components (such as alkaline anion exchange membranes).
Recently, Dr. Kumar Bugga was one of thirteen recipients nationwide to win a NASA Innovative Advanced Concepts (NIAC) award. Dr. Bugga will team with Michael Pauken at JPL, along with investigators from the California Institute of Technology and Liox, to develop a probe concept that can generate power from in-situ resources on Venus. The concept focuses on the generation of hydrogen from electrolysis at high altitudes using a solar array, storing it in a chemical hydride, utilizing it for altitude control in a balloon system, and generating power in a fuel cell at lower altitudes. Details can be found at: http://www.nasa.gov/feature/venus-interior-probe-using-in-situ-power-and-propulsion-vip-inspr
Previous studies have shown ISRU provides a viable pathway for supplying the fuel and oxygen for future Mars missions, by reducing readily available carbon dioxide from the Martian atmosphere. Ambient carbon dioxide reduction is likely to minimize or even eliminate the need to transport the large quantities of supplies from Earth to Mars. To successfully support these missions, however, large amounts of fuel and oxygen (e.g., 10 megatons per Martian year) will still be needed, necessitating development of high-volume approaches to carbon dioxide reduction. JPL has partnered with Caltech and UC San Diego to hold a Keck Institute for Space Studies workshop ("Addressing the Mars ISRU Challenge: Production of Oxygen and Fuel from CO2 using Sunlight") focused on the photo-electrochemical production of fuel (such as carbon monoxide) and oxygen from carbon dioxide on the Mars surface. Rather than using high temperature processes dependent on power generated from solar arrays, sunlight would be used directly with a catalytic process to affect the low temperature conversion of carbon dioxide to carbon monoxide and oxygen using large area deployable photoelectrochemical panels. More details can be found at: http://kiss.caltech.edu/new_website/workshops/isru/isru.html
Primary batteries have a long history in space exploration, going back to the very first JPL mission. The first spacecraft launched into orbit by the United States in 1958, Explorer 1, featured zinc-mercury oxide primary batteries. The Galileo Probe, which was released from the main spacecraft in 1995 to study the atmosphere of Jupiter, featured one of the first lithium-sulfur dioxide cells. The same heritage of primary battery chemistry was by the Huygens Probe to Titan in 2005. Primary batteries were also used with the first Mars Rover, Sojourner, in 1997. Thermal batteries are used extensively for critical, high power events such as entry, descent and landing.
As NASA and JPL mounts an aggressive exploration campaign to the Ocean Worlds, new primary chemistries are needed that can support long duration surface missions, where the solar flux is very low. The JPL Electrochemical Research, Technology, & Engineering Team is partnering with battery manufacturers to develop and qualify the next generation of aerospace primary batteries, to support the NASA exploration agenda. While most primary battery chemistries are designed to operate near Earth-ambient temperatures, JPL seeks to develop new electrodes, electrolytes and cell components that are radiation tolerant and can operate at extreme temperature.
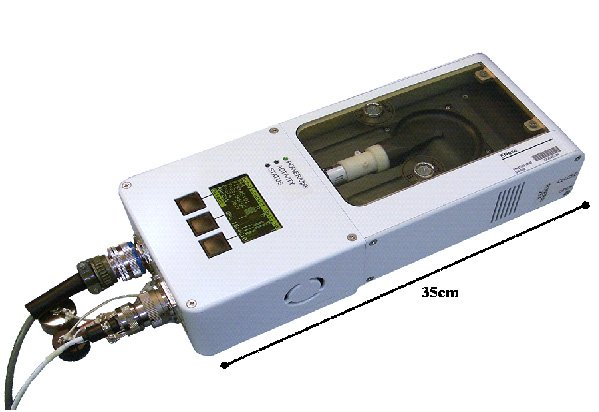
The Electrochemical Research, Technology, & Engineering Team is engaged in research and technology development of advanced sensors. For example, JPL's Electronic Nose (ENose) developed by the team is an event monitor for near real time air quality monitoring in crew habitat aboard the space shuttle/space station. This is an array–based sensing system which is designed to run continuously and to monitor for the presence of selected chemical species in the air at parts-per-million (ppm) to parts-per-billion (ppb) concentration ranges.
Its mission is to enable space exploration for the benefit of humankind by developing robotic spacecraft and instruments. There have been three phases of development of the JPL Electronic Nose. The third phase of development was designed to monitor spacecraft cabin air quality in near real-time. A technology demonstration of the Third Generation JPL ENose aboard the International Space Station (ISS) was performed in 2008-09 (below).
Analytes and targeted detection concentrations are discussed in previously published papers, as well as on the JPL ENose website (http://enose.jpl.nasa.gov). As a demonstration for use in medical applications, the JPL ENose was used in a pilot study to detect and differentiate brain cancer cells. In this proof-of-concept study, the JPL ENose distinguished the odor signatures of individual organs and glioblastoma and melanoma tumor cell lines. The JPL ENose, with proper sensor selection, can be applied to other earth-based environmental monitoring and medical applications.
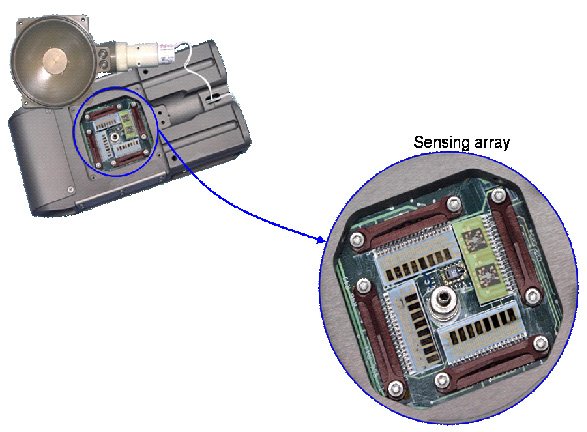
Sensing Chamber of the Third Generation JPL ENose sensor unit. Seen are sensor array on 4 chips (8 sensors per chip) optimized for target analytes. The sensing array consists of polymer-carbon, gold and palladium chloride films operating at 25-200 degrees Celsius on Si/alumina substrates. Sensors for temperature, pressure and relative humidity are also included in the sensing chamber.
Supercapacitors (also known as electrochemical capacitors or ultracapacitors) play an increasingly important role in a variety of high power and high reliability applications, providing energy storage for the public transportation sector (such as electric buses), regenerative braking energy capture in passenger vehicles, emergency aircraft back-up power supplies, medical defibrillators and wind turbine pitch control. Supercapacitors offer a higher specific energy than traditional electrolytic capacitors, with higher power densities and cycle lives relative to standard battery technologies. They are well suited for wide temperature operations where long cycle life is required, and JPL has pioneered the development of this technology for extreme environment applications.
Efforts at JPL encompass the full gamut of research and development activities, including materials development (in particular electrode and electrolyte materials), cell prototyping in coin, pouch and cylindrical formats, evaluation of both prototype and commercial available cells under relevant NASA conditions and ultimately infusion into flight. JPL regularly partners with external collaborators to further the technology for unique applications not met by existing options.
CL#25-4076